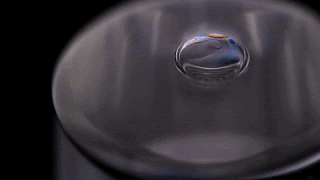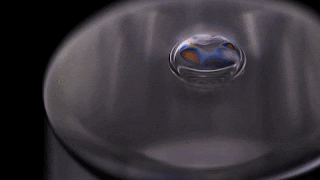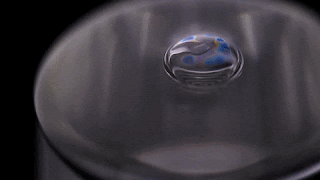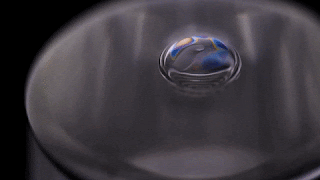
Bubbles don’t stick around in pure water. Surfactants are needed to stabilize the thin liquid film for longer than the blink of an eye. But that’s not necessarily the case for other liquids. As the video below shows, a bubble in isopropyl alcohol is quite stable. This is because of the alcohol’s volatility – its ability to evaporate easily.
As the alcohol in the bubble film evaporates, it cools the film, creating a difference in surface tension that pulls fresh alcohol up into the bubble film. It’s so efficient at pulling alcohol up that the alcohol can’t evaporate fast enough to use it all. Once the excess alcohol is heavy enough, it slides back down the side of the bubble. Overall, though, the process is enough to keep a bubble in pure isopropyl alcohol from rupturing for minutes to hours at a time.
Image and video
credit: M. Menesses et al.
No comments:
Post a Comment